
Long-acting Reversible Contraception (LARC)

LARCs Consists of:

Implants

Intra Uterine Systems (IUS)

Intra Uterine Devices (IUD)
LARCs are recommended as methods of contraception for women of all ages according to:
-
A) World Health Organization Medical Eligibility Criteria Guidelines.
-
B) Faculty of Family Planning & Reproductive Health Care Guidelines.
-
C) American College of Obstetricians and Gynaecologists
Annual cost of LARCs are lower than other methods
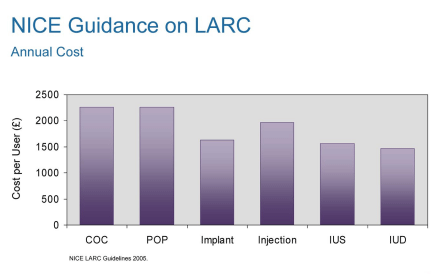
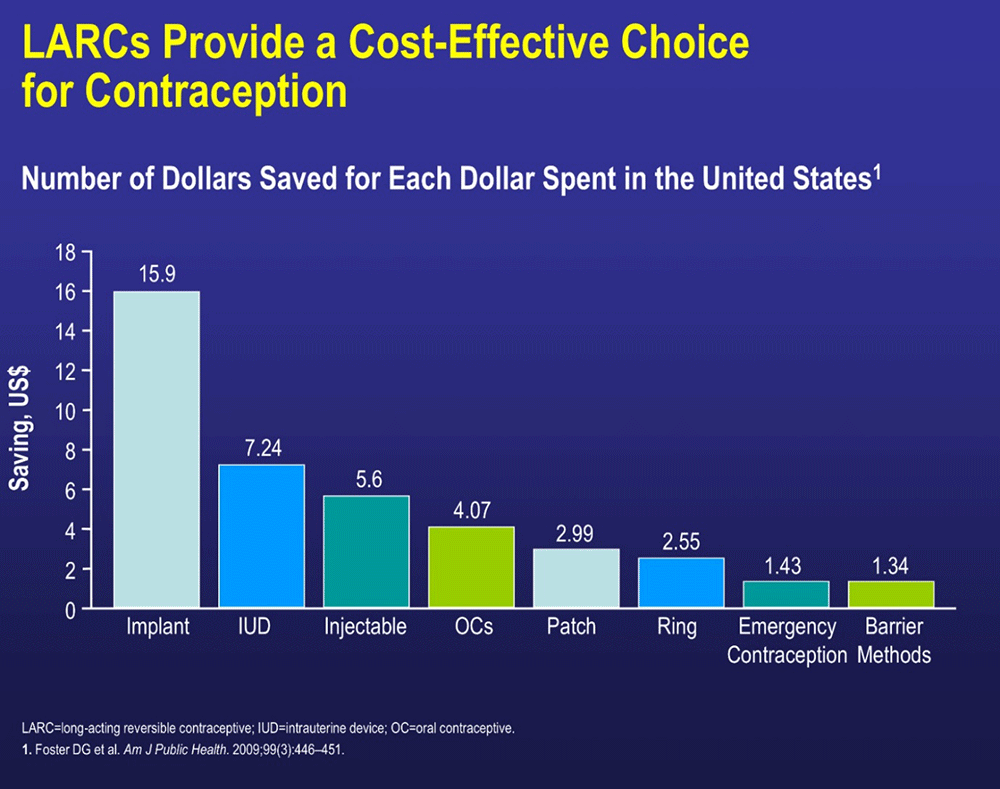
LARCs have the lowest typical use failure rate

The American College Of Obstetricians & Gynaecologist Committee Opinion
0ctober 2012
Intrauterine device & implants are the best reversible methods for preventing unintended pregnancy, rapid repeat pregnancy & abortion in young women.
American Academy of Paediatrics
POLICY STATEMENT ON CONTRACEPTION FOR ADOLESCENT 2014
Recommends That LARCS Methods Should Be Considered First Line Contraceptive Choices For Adolescents
Effectiveness of LARCs compared to other methods
THE CONTRACEPTIVE CHOICE PROJECT
-
New England Journal of Medicine May 2012
-
Prospective Cohort Study
-
August 2007 to September 2011
-
7,486 participants
-
Compared IUCD & Implants (LARC’s) vs Depoprovera, Pill, Patch or Ring
The Conclusions
-
Pill, patch or ring user are at 20 x higher risk of unintended pregnancy compared to LARC’s
-
Participants less than 21 years of age who used pills, patch or ring had 2x the risk of unintended pregnancy compare to those over 21 years old.
Intra uterine devices (IUD or IUCD)
Efficacy
-
Women should be advised of the very low failure rate associated with the use of IUCDs
-
The most effective system are the levonorgestrel intrauterine system ( LNG-IUS) and the T-shaped copper IUCDs with at least 380mm square copper and copper bands on the transverse arms
When can IUCDs be safely inserted?
-
A copper IUCD is effective immediately after insertion
-
It can be inserted at any time of the menstrual cycle if its reasonably certain the woman is not pregnant
-
Timing of insertion of a Copper IUCD did not have any significant effect on continuation, pregnancy & expulsion or bleeding and pain during insertion.
-
Copper IUCD can also be inserted as Emergency Contraception within 120 hrs of UPSI or up to 5 days after the earliest estimated date of ovulation.
-
LNG-IUS can be inserted up to day-7 of the menstrual cycle and any later insertions will require additional contraceptive precautions for 7 days
What assessment is needed prior to IUCD insertion?
-
Clinical assessment- Full medical history and assess eligibilty using WHOMEC
-
Additional investigations such as ultrasound, FBC etc only when indicated.
-
STI Risk Assessment:
-
Risk factors include:
-
Being sexually active and aged less than 25
-
having a new sexual partner in the last 3 months
-
having more than one sexual partner in the last one year
-
having a regular partner who has other sexual partners
-
A history of STIs
-
Attending as a previous contact of STI
-
alcohol/substance abuse
-
STI screen
-
Should be offered to all women who are identified to be at risk of STI when requesting an IUCD.
-
Minimum requirements:
-
Chamydia trachomatis testing
-
Detection of Chlamydia trachomatis (CT) and Neissseria gonorrhoea (NG) can be sent by endocervical swab via PCR methods.
-
Urine specimen are NO longer recommended for STI testing in women
-
Syphilis, HIV and Hep B screenings can be offered routinely
-
Intra uterine devices (IUD or IUCD) & Intra uterine systems (IUS)
Antibiotics Prophylaxis for Bacterial Endocarditis
-
National Institute for Health and Care Excellence (NICE) guidelines recommends that antibiotics prophylaxis is no longer offered routinely for defined interventional procedures.
-
Antibiotics prophylaxis are not routinely required for the insertion or removal of IUCD even in women with conditions where the risk of infective endocarditis may be increased
Benefits
1) Endometrial and other cancer protection
-
the 52mg LNG-IUS has been shown to provide endometrial protection from the stimulatory effects of estrogen.
-
Use of a Copper IUCD may be associated with a reduced risk of endometrial cancer and cervical cancer.
2) Dysmenorrhoea/Pelvic Pain
-
the LNG-IUS may reduce pain associated with primary dysmenorrhoea, endometriosis or adenomyosis.
3) Heavy menstrual bleeding
-
the LNG-IUS is effective in reducing menstrual blood loss and can be used in the management of heavy menstrual bleeding.
Health risks & concerns
-
Altered menstrual bleeding patterns is a common reason for discontinuation of Copper IUCDs and LNG-IUS
-
There is some evidence to suggest that bleeding patterns in IUCDs and LNG-IUS users tend to improve with time after insertion ( more than 3 months)
-
At one year, infrequent bleeding is usual with the LNG-IUS and some women may experience amenorrhoea.
Ectopic Pregnancy
-
Absolute risk of ectopic pregnancy is REDUCED in IUCD users compared to non-users
-
NICE estimate 1 in 1000 at 5 years
-
However, should a pregnancy occur in IUCD users, the likelihood of Ectopic is higher compared to non-users
-
Data are insufficient to determine the difference in ectopic rate of LNG-IUS users vs Copper IUCD users
-
Possibility of ectopic should be considered in IUCD users who are symptomatic and UPT positive.
Pelvic Inflammatory Disease (PID) & Perforation
-
PID appears to be linked to the insertion procedure and background risk of STIs
-
From review of randomised trials, there appears to be 6 fold increase risk of PID in the first 20 days, thereafter the risk remained low.
-
The overall risk of PID is low in IUCD users
-
The rate of uterine perforation is very low.
-
The rate is up to 2 per 1000 insertions and is approximately 6 fold higher in breastfeeding women.
How can of IUCD be safely inserted?
-
Training
-
The risk of perforation is related to the competence of healthcare professional.
-
The number of insertions done per year is related to the risk of perforation.
-
HCPs with experience of performing insertions less than 10 times in the span of 10 years period have a higher risk of inducing perforation among users.
-
Need for certification, recertification, trained and be conversant in anaphylaxis update and basic life support.
-
Valid consent should be taken prior to insertion.
-
Chaperone should be present.
Intervention to ease IUCD insertion
-
Nulliparity, No history of vaginal delivery, anxiety and lenght of time since last pregnancy or last menses are factors that may predict pain during insertion.
-
Cervical priming Agent- Cochrane review has shown that none of the priming agents reduce pain including misoprostol.
-
Prophylatic oral analgesia- Some evidence to show NSIADs may relieve post insertion pain BUT NOT pain during insertion.
-
Tissue forceps- Application facilitates IUCD insertion by stabilsing the cervix and reducing flexion angle of the uterus
-
Local Anaesthetics block can be offered for those that required cervical dilatation.
Practical Procedures for IUCD Insertions
-
Bimanual pelvic examination is essential for all women prior to IUCD insertion
-
HOWEVER, no evidence to suggest that cervical cleaning prior to IUCD insertion reduce subsequent pelvic infection
-
Sterile gloves are recommended during the procedure
-
Use of tissue forceps and uterine sound are also recommended
Managing Problems Associated with IUCD
-
Unscheduled bleeding
-
STIs
-
concurrent gynae causes
-
pregnancy
-
For LNG-IUS: Bleeding is common in the initial months and commonly settles without treatment, otherwise, COC can be tried up to 3 months
-
For Cu-IUCDs: NSAIDs can be considered and if ineffective, Tranexamic Acid
Non-Visible Threads
Non-Fundally placed IUCDs

-
Contraceptive efficacy may be compromised.
-
Decision to remove and replace a device is a matter of HCP's clinical judgement with careful consideration of user's personal and medical circumstances and to proceed after a discussion with the user
Pregnancy & presence of actinomyces-like organisms
-
Women who are pregnant with IUCD in situ-:
-
At greater risk of adverse pregnancy outcomes.
-
Spontaneous abortion, preterm delivery, septic abortion and chorioamnionitis
-
-
REMOVAL of IUCDs may help to improve outcome
-
Ectopic pregnancy need to be excluded
-
Actinomyces like organisms on pap smear in asymptomatic women DO NOT require removal of IUCDs
-
Suspected Pelvic Infection & Perforation
-
IUCDs removal is NOT routinely required in women with PID but should be remove if there is no response to treatment (Approx 72 hrs)
-
If there is any possilbilty of perforation, ultrasound scan and if indicated plain abdominal & pelvic x-ray should be arranged.
Emergency Contraception & Routine follow-up
-
The most effective EC methods
-
Can be inserted up to 120 hrs post UPSI or within 5 days of expected ovulation.
-
Routine follow up is not mandatory but can be advised after the first menses following insertion or 3 to 6 weeks later.
Removal and Replacement
Removal during licensed duration of use and alternative method chosen:
-
Removal of Cu-IUD at the end of licensed period; up to day-3: no additional precaution required.
-
Barrier contraception required for 7 days prior to removal/replacement if:
-
Removing Cu-IUD within the license period, in case re-insertion is not possible within the expected time-frame
-
Removing LNG-IUS at anytime
-
Removal Outside the licensed Period
-
Cu-IUD (containing more than 300mm^2 of Cu) if inserted AT OR AFTER the age of 40 can be retained until Menopause.
-
Women who had 52mg LNG-IUS inserted at the age of 45 or over can use it for 7 years or if amenorrhoeic until the menopause
IUCDs in specific populations
-
Nulliparous and adolescent women- IUCDs should not be restricted based on parity or age alone
-
Women with Cardiac disease- Vasovagal reaction is a serious risk with patients who have Single ventricle or Eisenmonger physiology.
-
IUCD use in such a population should involve cardiologist and insertion done in hospital setting.
-
Immucompromised patients- No evidence that IUCDs efficacy is reduced
-
Long term corticosteroids users- Need to consider increased steroid treatment proir to Insertion due to greater risk of cardiovascular collapse during insertion
Implants
Progestogen-only Implant
-
Single non-biodegradable, subdermal rod licensed for up to 3 years of use.
-
Each implant contains 68mg Etonogestrel (ENG)
-
The release rate from 60-70μg/day in weeks 5-6 to approximately 25-30μg/day at the end of third year.
-
Nexplanon NXT (detectable by Xray with addition of barium sulphate)
-
Primary mode of action is prevention of ovulation
-
Overall pregnancy rate is less than 1 in 1000 over 3 years.
Who is Eligible? WHO MEC 2015
Everyone!
EXCEPT:
-
Acute DVT/PE (3)
-
Positive (or unknown) antiphospholipid antibodies (3)
-
Unexplained Vaginal bleeding (before evaluation) (3)
-
Migraine with aura (3) (C)
-
Current and History of IHD or Stroke (3) (C)
When can it be safely inserted?
-
Up to day 5 of the menstrual cycle without the need for additional protection
-
At any time if it is reasonably certain that she is not pregnant with additional protection for the next 7 days.
Timing of Repeat Insertions
-
Women who return on schedule or earlier do not need additional contraceptive protection.
-
If after 3 years, additional contraceptive protection is needed, pregnancy testing and emergency contraception need to be considered if UPSI has occured.
Benefits and Risks of Progestogen-only Implant
-
Non-Contraceptive benefits:
-
Dysmenorrhoea and ovulatory pain may be alleviated.
-
May be beneficial in the treatment of endometriosis.
-
-
Health Concerns:
-
Little or no increased risk of venous thromboembolism, stroke or myocardial infarction.
-
No evidence of clinically significant adverse effects on bone mineral density.
-
-
Insufficient data to determine effect on breast cancer risk.
-
Absolute risk of ectopic is very low in view of ovulation suppression action.
Associated effects
-
Changes to bleeding pattern
-
Unscheduled bleeding in the first 3 months is common.
-
Fewer than 25% will have regular bleed. One third will have infrequent bleeding (Most common), One fifth will have amenoorrhea, and one quarter will have prolonged or frequent bleeding.
-
-
Changes in weight, mood & libido
-
No evidence for causal association.
-
-
Acne, Headache & Skin Atrophy
-
Acne can be worsened, improved or newly occurred.
-
Headache (no evidence of causation)
-
Skin atropy is a potential adverse effect of steroid use (case reports of skin atrophy at the site of subdermal implants).
-
Risks associated with Insertion or Removal Procedure
NON INSERTION:
-
Important to check for non-insertion and PALPATE skin after insertion.
DEEP INSERTION:
-
The correct position should be performed subcutaneously, just under the skin.
-
Deep insertion can happen due to inappropriate insertion technique.
-
May be diificult to remove/locate and greater potential for neurovascular injury, infection and scar formation
Removal
-
There is no need for additional precautions or abstinence prior to removal, providing it occurs no later than 3 years after insertion.
-
After removal , effective contraception is required immediately if pregnancy is not desired.
What follow up information?
-
No routine follow up is required but they can return anytime to discuss problems or change their contraceptive methods.
-
They can be advised to come back if:
-
Cannot feel the implant
-
any skin changes or pain around insertion site
-
they become pregnant
-
they develop any condition that may contraindicate the continuation of the method.
What might reduce the efficacy of Progestogen-only Implant?
A) Drug interactions:
-
Enzyme inducing drugs
-
Need to switch methods or additional contraception until 28 days after stopping the treatment.
B) Weight
-
Manufacturer states that earlier replacement can be considered in “ heavier” women but there is no direct evidence.
-
Obesity is a condition that there is No restriction on the use of Progestogen only Implant
-
No increase risk in pregnancy in women up to 149 kg.
Managing Problems Associated with Implant Use
-
IMPALPABLE IMPLANT
-
A woman with an impalpable implant should be advised to use additional precautions or abstinence until the presence of an implant is confirmed
-
-
Referral to an expert implant removal centre is recommended.
-
Location of an impalpable implant identified before exploratory surgery done.
-
High frequency linear ultrasound remains the recommended imaging.
-
Barium sulphate in the Nexplanon is radio opaque.
Problematic bleeding & pregnancy
-
After exclusion of other causes, women who experienced troublesome bleeding can be offered COC cyclically or continuously for 3 months.
-
Women with a continuing pregnancy should be advised to have the implant removed.
-
The implant is not known to be harmful in pregnancy.
References
1. Trussell J. Contraception; 2011;83:397-404
2. Long Acting Reversible Contraception
(NICE Clinical Guideline CG30) , Published October 2005, Last updated, September 2014.
3. WHO Medical Eligibility Criteria for Contraceptive Use, 2015
4. The American College of Obstetricians & Gynaecologists Committee Opinion, October 2012
5. Gina M. Secura; Jenifer E. Allsworth; Tessa Madden; Jennifer L.Mullersman; Jeffrey F.Peipert; The Contraceptive Choice Project: Reducing Barriers to Long Acting Reversible Contraception; American Journal of Obstetrics and Gynaecology,2010 Aug; 203(2)
6. Foster DG et al. Am J Public Health. 2009; 99(3):446-451
7. American Academy of Paediatrics, Policy Statement on Contraception for Adolescent, 2014
9. ACOG Practice Bulletin on Long Acting Reversible Contraception, Number 186, November 2017
Congratulations
You have completed the course
Would you like to take the Test Quiz?
Disclaimer: The information contained in this website is for general information purposes only. The information is provided by OGSS Online Contraceptive Course and while we endeavour to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.