
Contraception in High Risk Women

In women with medical disorders, especially in the developing world, the use of effective and safe contraception to prevent an unplanned pregnancy plays an important role in reducing maternal morbidity and mortality.
The Medical Eligibility Criteria (MEC) for Contraceptive Use (MEC) provides guidance on the safety and effective use of various contraceptive methods for women with medical conditions. The methods are divided into 4 categories based on each patient's specific health conditions and characteristics.
Category 1: No restriction to the use of method.
Category 2: The advantages outweigh the risks.
The method can be used but more careful follow up required.
Category 3: The risks outweight the advantages.
Not recommended unless other methods not available.
Category 4: Unacceptable health risk. Do not use.

In women with medical disorders, the risks of unplanned pregnancies must be considered to decide the most effective and acceptable method.
The use of long-acting reversible contraception (LARC) such as Cu-IUD, LNG-IUS or IMP,
is highly recommended if:
-
Unintended pregnancy increases the risk of exacerbating or aggravating the pre-existing medical conditions that could affect a woman’s health
-
The medication used have possible teratogenic effects.
Interaction with other medications
The use of enzyme-inducing drugs may reduce the contraceptive efficacy of hormonal contraception in the forms of oral/patch/ring/implant.
The use of DMPA, Cu-IUD or LNG-IUS are recommended during medication and for four weeks after.
Enzyme-inducing drugs that can affect hormonal contraception include:
Anti-epileptics:
carbamazepine, eslicarbazepine, fosphenytoin, oxcarbazepine, phenobarbital, phenytoin, primidone, rufinamide, topiramate
Anti-bacterials:
rifabutin, rifampicin
Anti-retrovirals:
ritonavir, ritonavir-boosted protease inhibitors, efavirenz, nevirapine
Others:
St John’s wort, modafinil, bosentan, aprepitant
Special Cautions & Possible Risks:
Venous Thromboembolic (VTE) Disease
The use of COC increases the risk of venous thromboembolic disease but the absolute risk is small.

** COC should not be used in the presence of venous thromboembolism (VTE) or arterial thromboembolism (ATE) conditions. Should any of the conditions appear for the first time during COC use, the product should be stopped immediately.
The risk of venous or arterial thrombotic/thromboembolic events increases with (but not limited to):
-
age
-
obesity
-
smoking
-
dyslipidaemia
-
a positive family history
-
immobilisation
-
hypertension
-
valvular heart disease
-
migraine
Age

Varicose veins
There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in venous thromboembolism.

Obesity
(BMI >= 30 for Caucasian population or
BMI >= 27.5 for Malaysian population)

Smoking
COC users who smoked and aged >=35 years have increased risk of cardiovascular disease, when compared to COC users who do not smoke. This risk of cardiovascular disease decreases 1-5 years after cessation of smoking.

Dyslipidaemia
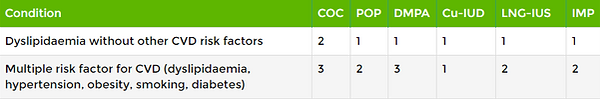
Cardiovascular diseases

Valvular and Congenital Heart Disease
-
Uncomplicated: UKMEC 1 for both Copper & LNG-IUCD
-
Complicated: (e.g. pulmonary hypertension, history of subacute bacterial endocarditis): UKMEC 2 for both Copper & LNG-IUCD
Uncomplicated cases are when:
-
No requirement for cardiac medication
-
Woman is asymptomatic
-
Cardiology review required annually or less
If in doubt, discuss with cardiologist
Prophylaxis against bacterial endocarditis is no longer indicated for women with Artificial Heart valves or previous endocarditis when inserting or removing IUCD.
However, this does not necessarily mean that there is no risk.
Neurological condition

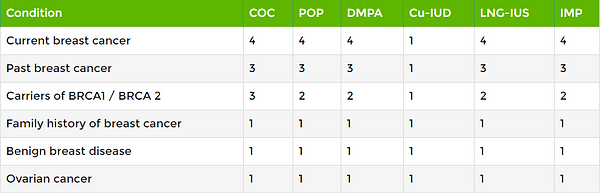
Breast and Ovarian diseases
The risk of breast cancer in women of reproductive age is small. The use of COC increases the risk of breast cancer but the absolute risk is small.
Uterine diseases
Hormonal contraception reduces the risk of endometrial cancer. The use of COC reduces the risk of uterine fibroids

Endometrial and Ovarian Cancer
-
COC use reduces the risk of developing endometrial cancer.
-
While awaiting treatment, women may use COC.
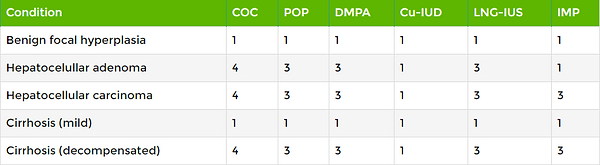
Liver diseases
In rare cases, benign liver tumours, and even more rarely, malignant liver tumours have been reported in users of COCs.
A hepatic tumour should be considered in the differential diagnosis when severe upper abdominal pain, liver enlargement or signs of intra-abdominal hemorrhage occur in women taking COCs,
** COC should not be used in presence of severe hepatic disease as long as liver function values have not returned to normal.
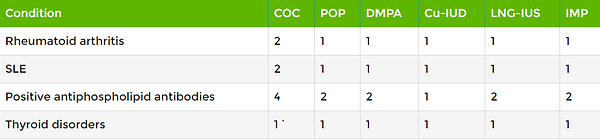
Autoimmune diseases
Methods of contraception for women with autoimmune disease should be recommended based on the MEC guidelines
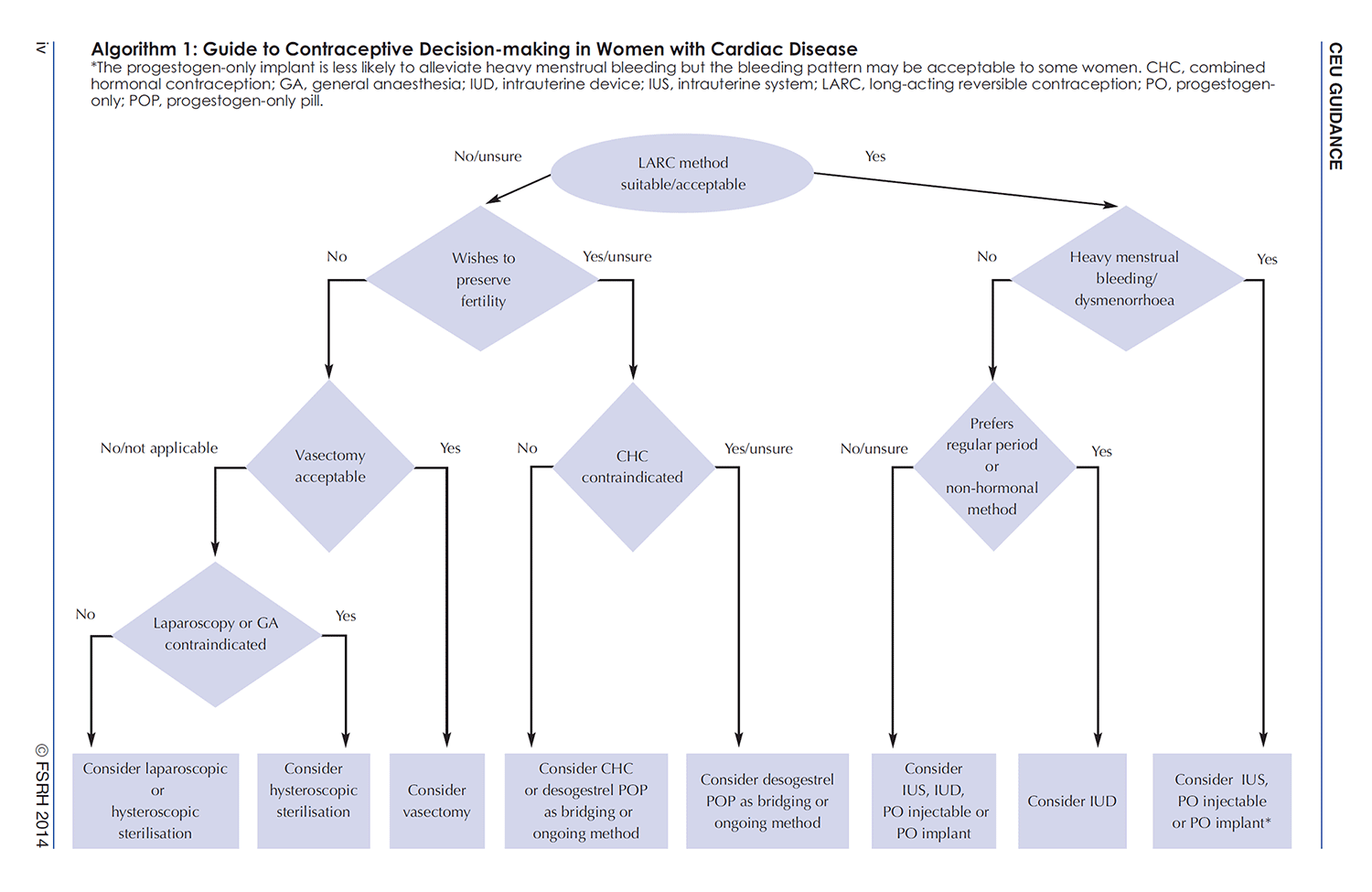
Guide to Contraceptive Decision Making in Women with Cardiac Disease
Cancer and sexually transmitted disease
-
Young people may be advised that COC's use is not associated with an overall increased risk of cancer.
-
Conversely, COC's use reduces the risk of ovarian, endometrial and colonic cancer.
-
The correct and consistent use of condoms should be advised to reduce the risks of STI’s
References
-
UK medical Eligibility Criteria for Contraceptive Use 2016
-
Contraceptive Choices for Women with Cardiac Disease- Faculty of Sexual and Reproductive Healthcare Clinical Guidance, Clinical Effectiveness Unit, June 2014
Congratulations
You have completed the course
Would you like to take the Test Quiz?
Disclaimer: The information contained in this website is for general information purposes only. The information is provided by OGSS Online Contraceptive Course and while we endeavour to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.